The ongoing effort to improve breast cancer detection involves a complex interplay of hardware innovations, computational refinement, and a deeper understanding of tissue biology, moving far beyond the simple two-dimensional X-ray image that has served as the screening cornerstone for decades. The inherent limitations of conventional mammography—most notably its reduced sensitivity in women with dense breast tissue, a characteristic that is both common and an independent risk factor for malignancy—have provided the impetus for a revolutionary shift in imaging technology. This evolution is characterized by a push toward volumetric, functional, and automated approaches that aim to reduce the ambiguity that historically plagued breast screening, increasing the true positive rate without an unacceptable rise in unnecessary biopsies. The newest modalities, often used adjunctively, seek to offer solutions where traditional screening falls short, providing clearer differentiation between benign fibroglandular tissue and early cancerous lesions. Furthermore, the integration of advanced data analysis, which is now possible due to the digitization of medical imaging, promises to redefine the role of the human interpreter in the diagnostic process.
The inherent limitations of conventional mammography—most notably its reduced sensitivity in women with dense breast tissue
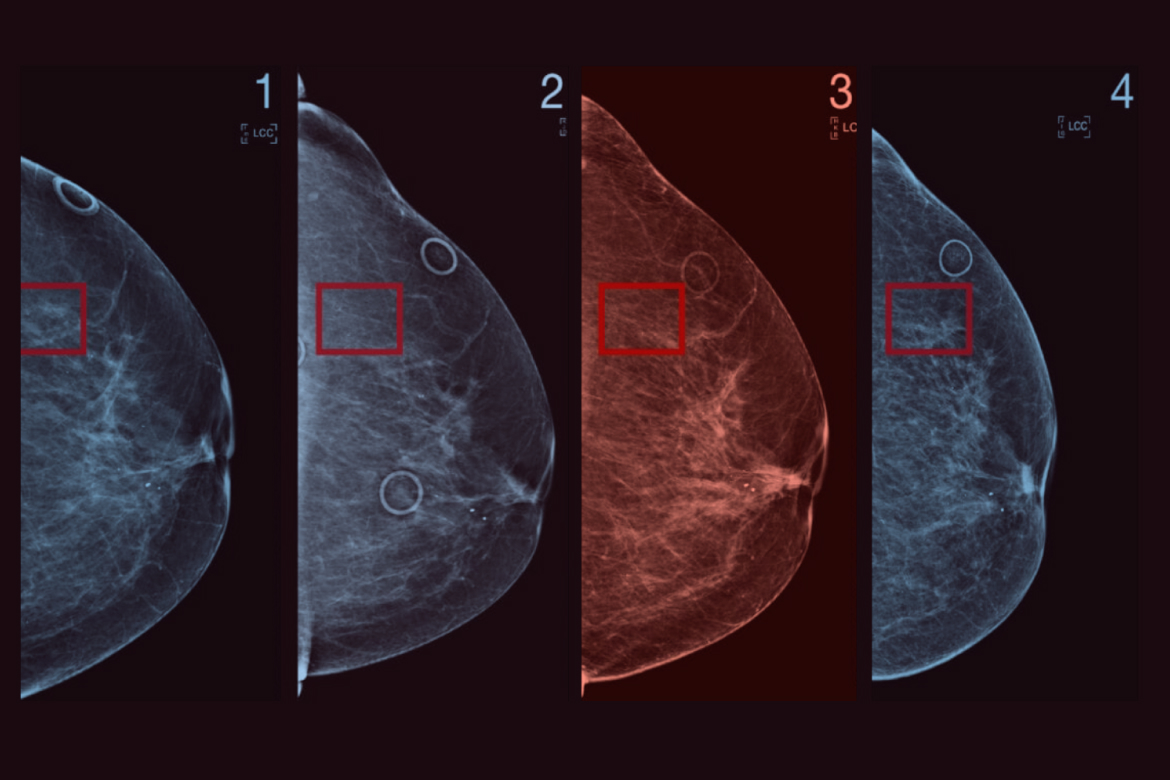
A significant hurdle in mass screening programs is the radiographic appearance of the breast parenchyma itself, which often mimics the very pathology being sought. “The inherent limitations of conventional mammography—most notably its reduced sensitivity in women with dense breast tissue” underscores a major clinical and technological bottleneck. Dense breasts, characterized by a higher proportion of fibroglandular tissue relative to fat, appear white on a mammogram. Since cancerous tumors also manifest as white densities, the malignancy can be effectively camouflaged, a phenomenon often described as “masking.” Digital Breast Tomosynthesis (DBT), frequently referred to as 3D mammography, emerged as the first widely adopted answer to this problem. By capturing a series of low-dose X-ray images from different angles and reconstructing them into a three-dimensional volume, DBT allows the radiologist to scroll through thin slices of breast tissue, dramatically reducing the effect of overlapping structures that obscure small cancers in dense breasts.
By capturing a series of low-dose X-ray images from different angles and reconstructing them into a three-dimensional volume
Digital Breast Tomosynthesis represents a fundamental upgrade to the anatomical visualization capabilities of X-ray-based imaging. “By capturing a series of low-dose X-ray images from different angles and reconstructing them into a three-dimensional volume” describes the core technical mechanism that has improved cancer detection rates, particularly for smaller, invasive tumors. While DBT has significantly lowered recall rates and increased the detection of invasive cancers compared to standard 2D digital mammography, it still relies on morphology—the shape and structure of the tissue—for diagnosis. This is where functional imaging modalities come into play, offering a physiological view of the tissue by assessing blood flow and metabolic activity, which are fundamentally altered in malignancy. Techniques such as Contrast-Enhanced Mammography (CEM) and Molecular Breast Imaging (MBI) are gaining traction by exploiting the increased vascularity and high metabolic demand of growing tumors.
Techniques such as Contrast-Enhanced Mammography (CEM) and Molecular Breast Imaging (MBI) are gaining traction
The clinical utility of adding functional data to morphological assessment is rapidly changing the diagnostic pathway for high-risk and dense-breasted populations. “Techniques such as Contrast-Enhanced Mammography (CEM) and Molecular Breast Imaging (MBI) are gaining traction” signals a shift towards specialized, yet increasingly accessible, adjunct screening tools. CEM involves administering an iodine-based contrast agent intravenously, which rapidly highlights areas of increased blood flow (angiogenesis) within the breast, a hallmark of aggressive tumor growth. MBI, in contrast, uses a technetium-based radiotracer that is preferentially taken up by metabolically active cancer cells, with gamma cameras then capturing the resulting signal. Both methods provide excellent sensitivity, often rivaling that of Magnetic Resonance Imaging (MRI), but at a lower cost and with greater convenience, making them powerful tools for clarifying ambiguous findings or screening women for whom standard mammography is insufficient.
Both methods provide excellent sensitivity, often rivaling that of Magnetic Resonance Imaging (MRI)
The development of CEM and MBI provides necessary alternatives for achieving high diagnostic performance outside of the more complex and expensive MRI environment. “Both methods provide excellent sensitivity, often rivaling that of Magnetic Resonance Imaging (MRI)” places these new techniques in the context of the current gold standard for high-risk screening. MRI, utilizing powerful magnetic fields and radio waves, remains an exceptionally sensitive tool, especially when paired with a gadolinium-based contrast agent, which highlights abnormal vascularity with high spatial resolution. However, its high cost, limited availability, and potential for false positive results necessitate its use being generally confined to women with known high-risk genetic mutations, a strong family history, or a history of prior chest radiation. Abbreviated breast MRI protocols are now being investigated to streamline the process, aiming to retain diagnostic performance while reducing scan time and overall cost.
Abbreviated breast MRI protocols are now being investigated to streamline the process
The drive for efficiency and accessibility is critical across all advanced imaging modalities, prompting the exploration of time-compressed diagnostic sequences. “Abbreviated breast MRI protocols are now being investigated to streamline the process” reflects the ongoing effort to make highly effective, yet resource-intensive, techniques practical for broader application. While technological advancements in hardware are essential, the most disruptive progress is arguably occurring in the domain of image interpretation itself. The sheer volume and complexity of data generated by 3D imaging, CEM, and MRI now require computational assistance for human readers to maintain accuracy and efficiency. This necessity has propelled the development and clinical integration of Artificial Intelligence (AI) and Machine Learning (ML) algorithms, which are beginning to reshape the entire workflow of breast cancer screening and diagnosis.
The sheer volume and complexity of data generated by 3D imaging, CEM, and MRI now require computational assistance
The cognitive burden placed on radiologists by modern imaging techniques mandates a technological partnership to effectively manage the data. “The sheer volume and complexity of data generated by 3D imaging, CEM, and MRI now require computational assistance” serves as the primary justification for the rapid adoption of AI in this field. Deep learning models, particularly Convolutional Neural Networks (CNNs), are trained on millions of images to identify subtle patterns—such as early microcalcifications or architectural distortions—that are often too faint or complex for the fatigued human eye to consistently notice. These AI systems function in two primary ways: as a “second reader” to increase the sensitivity of the screening process, or as a triage tool to flag high-risk cases for immediate human review, thereby increasing the efficiency of the entire screening program and reducing reading times.
These AI systems function in two primary ways: as a “second reader” to increase the sensitivity of the screening process
The core clinical application of AI currently centers on enhancing the reliability and consistency of the interpretation process. “These AI systems function in two primary ways: as a “second reader” to increase the sensitivity of the screening process” describes the immediate, measurable benefit. By acting as a tireless and objective interpreter, the algorithm can reduce the potential for human error inherent in double-reading protocols. Furthermore, AI is not confined to image interpretation alone. Machine learning models are also being developed for risk stratification, analyzing a patient’s mammographic data in conjunction with clinical history and genetics to predict their probability of developing cancer between screening intervals (interval cancers), or even within the next several years. This capacity promises a shift toward truly personalized screening schedules, moving away from uniform, age-based recommendations.
Machine learning models are also being developed for risk stratification
Moving beyond immediate diagnosis, computational models are extending their utility into proactive patient management. “Machine learning models are also being developed for risk stratification” introduces the concept of using AI to inform personalized screening protocols. The ability to calculate individual risk profiles with greater precision allows clinicians to tailor the frequency and modality of screening. A patient with very dense breasts and a high ML-derived risk score may be fast-tracked for alternating mammography and abbreviated MRI, while a patient with fatty breasts and a low risk score might confidently adhere to standard biennial screening. This paradigm fundamentally alters the one-size-fits-all approach that has defined population-based screening for decades, offering a path to greater resource efficiency and reduced patient anxiety associated with unnecessary procedures.
This capacity promises a shift toward truly personalized screening schedules
The trajectory of breast cancer detection is clearly moving toward highly individualized and integrated diagnostic systems. “This capacity promises a shift toward truly personalized screening schedules” encapsulates the ultimate goal of combining technological advances with data-driven decision-making. However, the future of detection is not solely image-based. The rise of liquid biopsy and circulating tumor DNA (ctDNA) analysis is poised to become another revolutionary layer. These non-invasive blood tests aim to detect minute fragments of cancerous genetic material shed by tumors into the bloodstream. While still largely experimental in the screening context, the promise of a simple blood test to identify cancer in its earliest, non-metastatic stages could eventually supplement, or even partially supplant, the current suite of imaging techniques, offering an unprecedented level of early detection for all populations.
The rise of liquid biopsy and circulating tumor DNA (ctDNA) analysis is poised to become another revolutionary layer
Exploring non-radiological methods represents a departure from reliance on physical visualization, aiming for biological detection at the molecular level. “The rise of liquid biopsy and circulating tumor DNA (ctDNA) analysis is poised to become another revolutionary layer” signifies the expansion into molecular diagnostics. The challenge here lies in achieving sufficient sensitivity to detect ctDNA from very small, early-stage tumors and, critically, in differentiating it from background “noise” of non-cancerous cellular turnover. Successfully integrating these molecular biomarkers with advanced imaging and AI-driven risk models will be the defining characteristic of next-generation breast cancer screening. This convergence of hardware, computation, and molecular biology is driving a complex and exciting transformation, ultimately geared toward identifying malignancies when they are most treatable, without excessively burdening patients or healthcare systems with overdiagnosis.