The function of the oncologic surgeon in the modern context of cancer care is fundamentally misunderstood if viewed only as the specialist who excises the tumor. While the removal of malignant tissue remains a core competency, their actual influence spans the entire patient journey, beginning with the initial diagnostic uncertainty and extending through complex planning, intraoperative judgment calls, and long-term survivorship management. The surgical oncologist acts as a pivotal decision-maker, often the first specialist to lay hands on the disease and establish its definitive pathological status. Their expertise in the natural history of a vast array of malignancies allows them to anticipate patterns of spread and local recurrence that guide the necessary extent of resection, balancing the imperative for complete tumor eradication with the crucial goal of preserving function and quality of life. This intricate balancing act is what truly defines the surgical oncologist’s contemporary contribution to a patient’s treatment strategy, a contribution which often involves steering the entire multidisciplinary team through the most challenging management dilemmas.
The entire patient journey, beginning with the initial diagnostic uncertainty and extending through complex planning, intraoperative judgment calls, and long-term survivorship management.
The initial phase of a cancer diagnosis relies heavily on the surgical oncologist’s ability to obtain adequate, representative tissue for pathological and molecular analysis. This is frequently accomplished through biopsy procedures, which require both technical skill to access the tumor safely and anatomical insight to avoid compromising future curative operations. A poorly planned or executed initial biopsy can track tumor cells, complicate subsequent resections, or even prematurely preclude a patient from an otherwise viable therapeutic pathway. Following the confirmation of malignancy, the surgeon collaborates with radiologists and pathologists to review all imaging and diagnostic reports, a process that is absolutely critical in precisely determining the extent and stage of the cancer. This collaboration is instrumental in determining if the cancer is localized enough for surgery to be the primary curative modality, or if neoadjuvant therapy (such as chemotherapy or radiation) is required first to shrink the tumor and improve the chances of a clean surgical margin. Their input at this stage is the linchpin that locks in the most appropriate initial course of action, guiding the patient and the rest of the care team toward an individualized strategy.
A poorly planned or executed initial biopsy can track tumor cells, complicate subsequent resections, or even prematurely preclude a patient from an otherwise viable therapeutic pathway.
The planning of the surgical intervention is seldom a straightforward decision in isolation; rather, it is the result of a rigorous, multidisciplinary team (MDT) discussion where the surgical oncologist’s voice carries the weight of practical anatomical possibility and curative probability. This team—comprising medical oncologists, radiation oncologists, pathologists, and specialty nurses—convenes to collaboratively develop an individualized treatment plan. The surgeon must clearly articulate the potential for achieving an R0 resection (complete microscopic removal of the tumor with clear margins) versus the risks of morbidity or functional impairment associated with the required extent of tissue removal. Cases involving complex anatomical locations, such as head and neck, retroperitoneal, or pelvic malignancies, are particularly challenging, as they necessitate a trade-off between survival and functional outcome. The MDT environment serves as a critical check and balance, where different specialties offer their perspectives on sequencing the treatment: should surgery come first, or should it be preceded by systemic or regional therapies to optimize the tumor’s responsiveness and resectability?
The surgeon must clearly articulate the potential for achieving an R0 resection versus the risks of morbidity or functional impairment associated with the required extent of tissue removal.
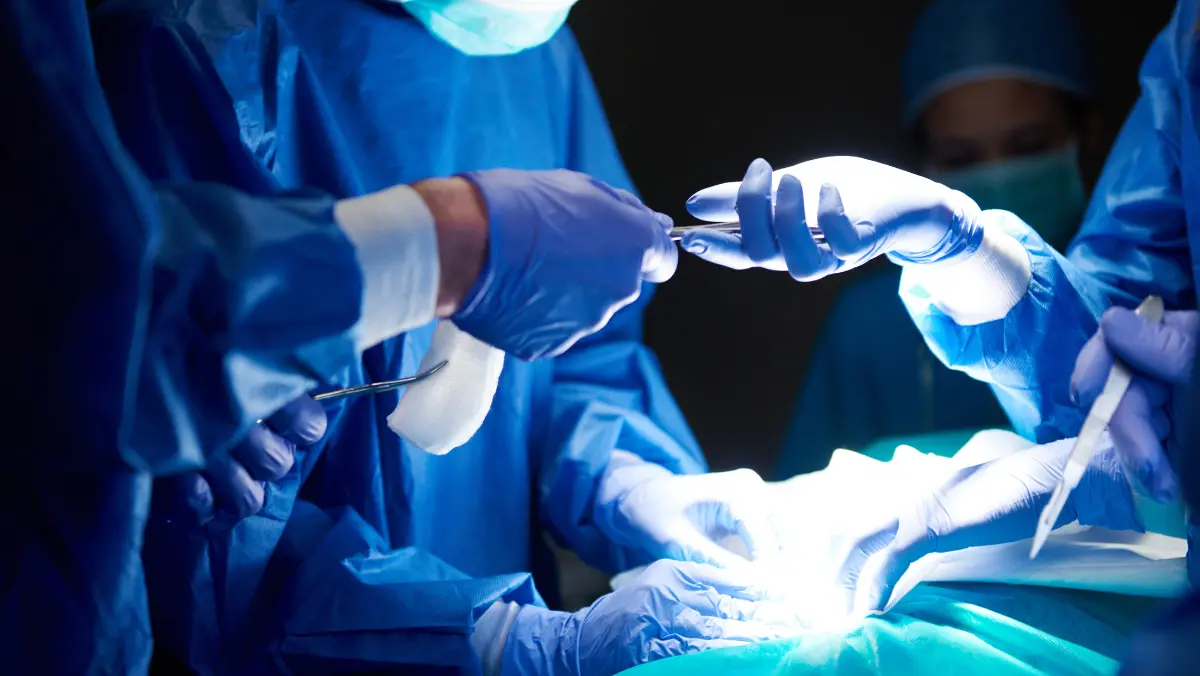
During the operation itself, the oncologic surgeon transforms from a planner into a rapid, decisive technician, where intraoperative decision-making is continuous and often deviates from the preoperative map. Despite extensive imaging, the full scope of the disease, including subtle invasions or unexpected nodal involvement, is frequently only unveiled once the surgical field is exposed. The surgeon’s deep training allows for the on-the-spot assessment of margin adequacy, a crucial factor often confirmed by consulting a pathologist who may be present in the operating room for frozen section analysis. Their primary goal remains the complete eradication of the macroscopic disease with a buffer of healthy tissue—the clear margin—a requirement paramount to minimizing the risk of local recurrence. The ability to navigate dense, previously treated or anatomically critical areas, choosing the correct plane of dissection that spares vital structures, is where the surgical oncologist’s dedicated expertise in the three-dimensional spread of cancer becomes absolutely indispensable.
The surgeon transforms from a planner into a rapid, decisive technician, where intraoperative decision-making is continuous and often deviates from the preoperative map.
The increasing adoption of minimally invasive techniques, including laparoscopic and robotic surgery, has significantly changed the oncologic surgeon’s technical repertoire. These advancements are not merely about making smaller incisions; they represent a fundamental shift towards enhanced precision, reduced blood loss, and faster patient recovery, allowing a quicker transition to any necessary adjuvant therapies. Robotic surgery, in particular, offers the surgeon improved dexterity, three-dimensional high-definition visualization, and tremor filtration, which are distinct advantages when performing intricate dissections in constrained spaces, such as deep within the pelvis for rectal cancer or in the chest for lung lesions. However, the successful application of these technologies requires specialized training and a judicious selection of patients, as not every tumor or patient profile is suitable for a minimally invasive approach. The surgical oncologist must maintain a comprehensive skill set, capable of seamlessly converting to a traditional open procedure if unexpected intraoperative findings or technical complications dictate a change in strategy.
The increasing adoption of minimally invasive techniques, including laparoscopic and robotic surgery, has significantly changed the oncologic surgeon’s technical repertoire.
The operative field extends beyond the removal of the primary tumor to include the management of regional lymph nodes, which are often the initial sites of microscopic disease spread. Lymph node dissection is a critical staging procedure, providing pathologists with the necessary information to determine the extent of metastasis, thereby influencing the prognosis and the need for adjuvant systemic therapy. For many solid tumors, such as breast cancer and melanoma, the technique of sentinel lymph node biopsy has revolutionized this aspect of surgery. This less invasive procedure identifies and removes only the first few lymph nodes to which the tumor is likely to spread, sparing the majority of lymph nodes if they are clear of malignancy. This focused approach significantly reduces the risk of long-term complications, such as lymphedema, demonstrating the surgical oncologist’s constant consideration for improving the patient’s long-term functional outcome alongside curative intent.
Lymph node dissection is a critical staging procedure, providing pathologists with the necessary information to determine the extent of metastasis, thereby influencing the prognosis.
In cases of advanced or metastatic disease, the surgical oncologist’s role often pivots from curative intent to the complex domain of palliative and cytoreductive surgery. While a complete cure may not be attainable, surgery can still dramatically improve the patient’s quality of life by relieving intractable symptoms, such as bowel obstruction, severe pain, or bleeding. Furthermore, in selected metastatic settings, such as liver or lung metastases from colorectal cancer, the surgical removal of all identifiable disease (cytoreduction) can significantly prolong survival when combined with systemic therapies. These operations, often referred to as “metastasis-directed treatments,” are highly specialized and require an exceptionally close working relationship with the medical oncologist to ensure the timing of surgery integrates optimally with the patient’s chemotherapy or immunotherapy schedule. The decision to undertake such an extensive, non-curative procedure is one of the most ethically and technically demanding in oncology, requiring detailed patient-physician communication about realistic expectations and potential benefits.
The surgical oncologist’s role often pivots from curative intent to the complex domain of palliative and cytoreductive surgery.
The responsibility of the oncologic surgeon does not conclude when the final suture is placed; it transitions into the critical phase of postoperative care and surveillance. They must manage the immediate surgical recovery, monitoring for complications that are often compounded by the patient’s underlying cancer status or prior neoadjuvant treatment. Beyond the initial recovery, the surgeon takes an active role in the long-term follow-up, establishing surveillance schedules that often involve serial imaging and tumor marker checks to detect recurrence at its earliest, most treatable stage. This ongoing relationship ensures continuity of care, as the surgical oncologist is uniquely positioned to interpret changes in the surgical site or regional anatomy, providing the clinical context needed to correctly evaluate subsequent scans and test results. They often serve as a continuous consultant to the rest of the care team, re-entering the treatment plan should a local or locoregional recurrence necessitate further surgical intervention.
The surgeon takes an active role in the long-term follow-up, establishing surveillance schedules that often involve serial imaging and tumor marker checks.
Looking toward the future, the surgical oncology field is rapidly integrating personalized medicine strategies and emergent technologies, particularly in the realm of molecular profiling and immunotherapy. Surgeons are increasingly involved in obtaining tumor samples not just for pathology, but for comprehensive genomic analysis that can identify targets for specific drugs. Furthermore, there is a growing involvement in combining surgery with novel agents, where the surgical debulking of the tumor can potentially enhance the efficacy of subsequent immunotherapy. The development of Artificial Intelligence (AI) tools for enhancing diagnostic accuracy and staging is also set to transform the preoperative planning phase, offering the potential for more precise risk assessment and surgical roadmapping. The surgical oncologist must stay ahead of these advances, integrating new protocols and technologies into their practice while maintaining the core principles of meticulous technique and sound clinical judgment, navigating the complex interplay between molecular biology and operative mechanics.
The surgical oncology field is rapidly integrating personalized medicine strategies and emergent technologies.
The ultimate measure of the oncologic surgeon’s effectiveness extends far beyond the technical success of the operation; it lies in their ability to lead an integrated, patient-focused approach, ensuring that every therapeutic decision—from the initial biopsy to the most complex recurrence management—is aligned with the patient’s best possible long-term survival and quality of life. They are the indispensable specialists who possess the unique blend of anatomical mastery, oncological knowledge, and decisive judgment necessary to physically intervene in the disease process, fundamentally altering the trajectory of a cancer diagnosis.
The ultimate measure of the oncologic surgeon’s effectiveness extends far beyond the technical success of the operation.
The modern surgical oncologist orchestrates precise, individualized cancer treatment strategies, translating complex molecular data into decisive curative action.